Jan 30, 2023Surface Tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface
Joints Terminology – Geology In
Place the penny, heads up, on top of a paper towel. 3. Hold your dropper about 1-inch above the penny and add drops of water to the surface of the penny until it overflows. 4. Record the number of drops of water the surface of the penny can hold in the table on the next page under the column labeled “Run 1.”. 5.

Source Image: studypool.com
Download Image
Identify the true statements about surface tension. a. Molecules along the surface of a liquid behave differently than those in the bulk liquid. b. Mercury has a lower surface tension than water. c. Cohesive forces attract the molecules of the liquid to one another. d. Surface tension increases as the temperature of the liquid rises. e.

Source Image: pubs.acs.org
Download Image
Chinese Whispers 珠落玉盤 5 days agoSurface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it.The molecules in a drop of water, for example, attract each other weakly. Water molecules well inside the drop may be thought of as being attracted equally in all directions by the surrounding molecules.

Source Image: numerade.com
Download Image
Identify The True Statements About Surface Tension.
5 days agoSurface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it.The molecules in a drop of water, for example, attract each other weakly. Water molecules well inside the drop may be thought of as being attracted equally in all directions by the surrounding molecules. Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged.. At liquid-air interfaces, surface tension results from the greater attraction of liquid
SOLVED: Identify the true statements about surface tension. Molecules along the surface of a liquid behave differently than those in the bulk liquid. Mercury has a lower surface tension than water. Cohesive
Identify the true statements about surface tension. a. Molecules along the surface of a liquid behave differently than those in the bulk liquid. b. Mercury has a lower surface tension than water. c. Cohesive forces attract the molecules of the liquid to one another. d. Surface tension increases as the temperature of the liquid rises. e. All-atmospheric fabrication of Ag–Cu core–shell nanowire transparent electrodes with Haacke figure of merit >600 × 10–3 Ω−1 | Scientific Reports
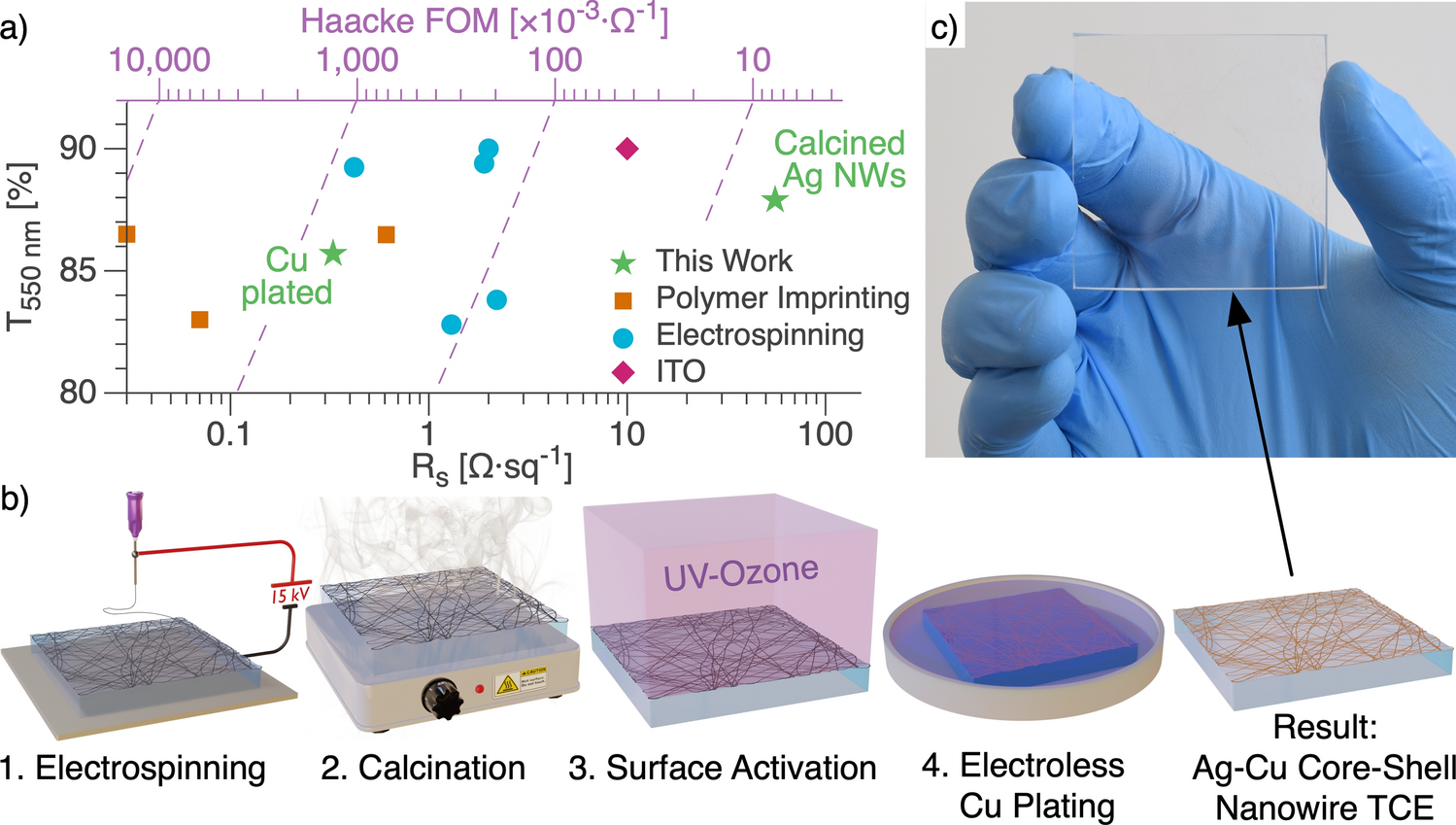 Download Image
Download ImageSolved Which of the following statements is true regarding | Chegg.com Identify the true statements about surface tension. a. Molecules along the surface of a liquid behave differently than those in the bulk liquid. b. Mercury has a lower surface tension than water. c. Cohesive forces attract the molecules of the liquid to one another. d. Surface tension increases as the temperature of the liquid rises. e.

Source Image: chegg.com
Download Image
Joints Terminology – Geology In Jan 30, 2023Surface Tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface
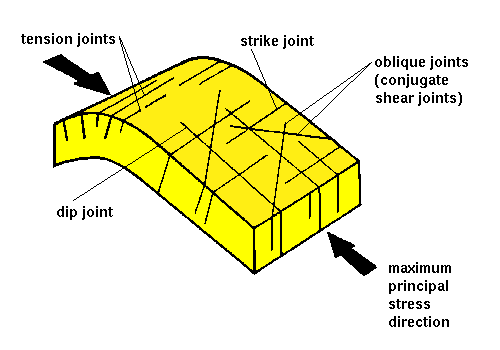
Source Image: geologyin.com
Download Image
Chinese Whispers 珠落玉盤 Identify the true statements about surface tension. a. Molecules along the surface of a liquid behave differently than those in the bulk liquid. b. Mercury has a lower surface tension than water. c. Cohesive forces attract the molecules of the liquid to one another. d. Surface tension increases as the temperature of the liquid rises. e.

Source Image: facebook.com
Download Image
Light-driven biological actuators to probe the rheology of 3D microtissues | Nature Communications Sep 21, 2022It is also responsible for the beading up of water droplets on a freshly waxed car because there are no attractions between the polar water molecules and the nonpolar wax. Figure 13.6.2 13.6. 2: (A) Molecules at the surface of a liquid are pulled downwards into the liquid, creating a tightened surface. (B) Surface tension allows a paper clip to

Source Image: nature.com
Download Image
Mechanosensation of Tight Junctions Depends on ZO-1 Phase Separation and Flow – ScienceDirect 5 days agoSurface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it.The molecules in a drop of water, for example, attract each other weakly. Water molecules well inside the drop may be thought of as being attracted equally in all directions by the surrounding molecules.

Source Image: sciencedirect.com
Download Image
State of the art of indigenous languages in research: a collection of selected research papers Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged.. At liquid-air interfaces, surface tension results from the greater attraction of liquid

Source Image: unesdoc.unesco.org
Download Image
Solved Which of the following statements is true regarding | Chegg.com
State of the art of indigenous languages in research: a collection of selected research papers Place the penny, heads up, on top of a paper towel. 3. Hold your dropper about 1-inch above the penny and add drops of water to the surface of the penny until it overflows. 4. Record the number of drops of water the surface of the penny can hold in the table on the next page under the column labeled “Run 1.”. 5.
Chinese Whispers 珠落玉盤 Mechanosensation of Tight Junctions Depends on ZO-1 Phase Separation and Flow – ScienceDirect Sep 21, 2022It is also responsible for the beading up of water droplets on a freshly waxed car because there are no attractions between the polar water molecules and the nonpolar wax. Figure 13.6.2 13.6. 2: (A) Molecules at the surface of a liquid are pulled downwards into the liquid, creating a tightened surface. (B) Surface tension allows a paper clip to